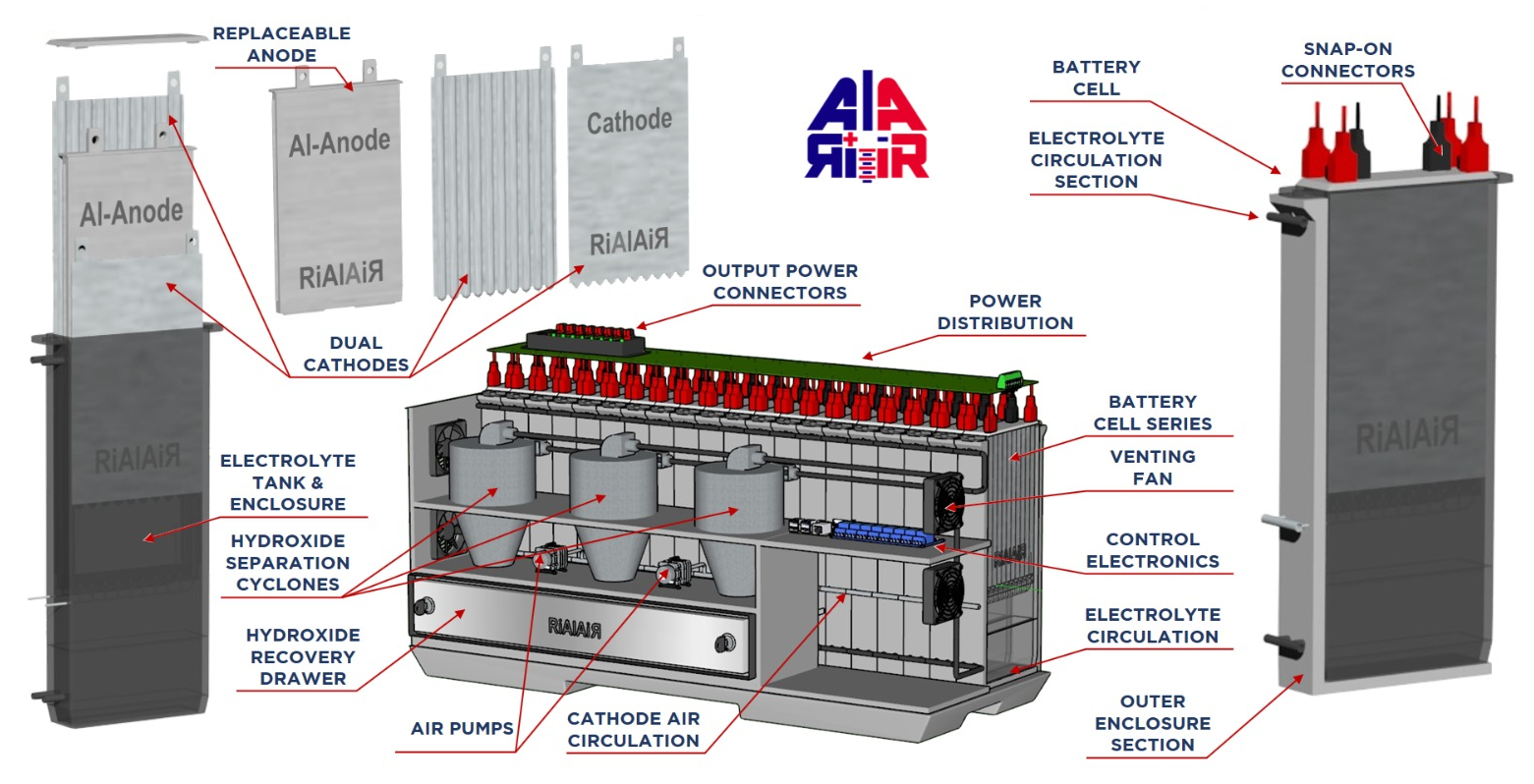
The Al-Air cell is generically composed of the following elements: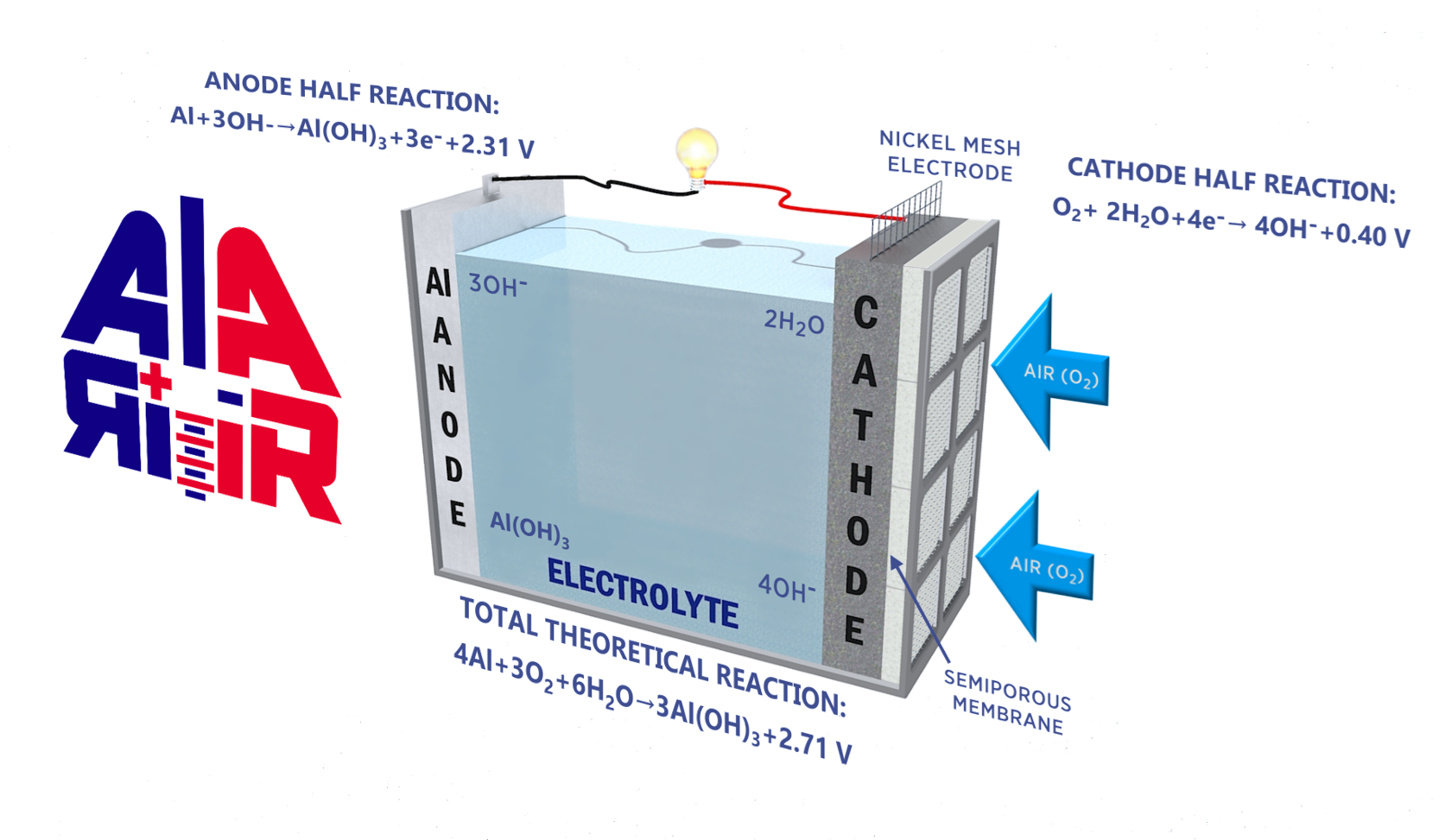
-
- An aluminium negative electrode (anode), giving up electrons with oxidation, that will consist of Al, either pure or as an alloy.
- A positive electrode (cathode), accepting electrons and enabling oxygen transport and reduction, often by means of a catalyst. The materials could be among others, carbon, graphite, manganese dioxide, PTFE, titanium dioxide, nanotube arrays and graphene, with a nickel or stainless steel, conducting electrode .
- A suitable electrolyte for the transfer of ions between the anode and the cathode. This is typically an aqueous alkaline solution consisting of sodium hydroxide (NaOH), potassium hydroxide (KOH) or neutral such as sodium chloride (NaCl).
The RiAlAiR Battery Components
Aluminium–Air (Al–Air) batteries are primary cells, thus non-electrically rechargeable. The aluminium anode immersed in a water-based electrolyte is consumed by its reaction with atmospheric oxygen (the cathode). Aluminium–Air batteries produce electricity from the reaction of the oxygen in the air with the aluminium anode.

The by-product of this reaction is hydrated Aluminium oxide (Aluminium Hydroxide). Once all the Aluminium reacts to form Aluminium Hydroxide, the battery will no longer produce electricity.
The good news is that not only it is possible to recharge mechanically the battery by replacing the exhaust anodes with new Aluminium anodes, but it is also possible to recycle the Aluminium Hydroxide, so that the cycle is 100% environmentally friendly and no emissions are released into water or air.
Al-Air batteries have one of the highest energy densities of all batteries because part of the reactants come from the air. Since Aluminium is a lightweight metal and the cathode reactant, oxygen, does not have to be stored in the battery, an Al-Air battery is considerably lighter than a comparable Lithium-Ion battery.